Abstract
Following the discovery of high-temperature superconductivity in the La–H system, we studied the formation of new chemical compounds in the barium-hydrogen system at pressures from 75 to 173 GPa. Using in situ generation of hydrogen from NH3BH3, we synthesized previously unknown superhydride BaH12 with a pseudocubic (fcc) Ba sublattice in four independent experiments. Density functional theory calculations indicate close agreement between the theoretical and experimental equations of state. In addition, we identified previously known P6/mmm-BaH2 and possibly BaH10 and BaH6 as impurities in the samples. Ab initio calculations show that newly discovered semimetallic BaH12 contains H2 and H3– molecular units and detached H12 chains which are formed as a result of a Peierls-type distortion of the cubic cage structure. Barium dodecahydride is a unique molecular hydride with metallic conductivity that demonstrates the superconducting transition around 20 K at 140 GPa.
Similar content being viewed by others
Introduction
In recent years, the search for new hydride superconductors with TC close to room temperature attracts great attention of researchers in the field of high-pressure materials science. Variation of pressure opens prospects of synthesis of novel functional materials with unexpected properties1. For example, according to theoretical models2,3,4,5, compression of molecular hydrogen over 500 GPa should lead to the formation of an atomic metallic modification with TC near room temperature. Pressures of 420–480 GPa were achieved in experiments with toroidal diamond anvil cells6; however, for conventional high-pressure cells with a four-electrode electric setup, pressures above 200 GPa remain challenging.
In 2004, Ashcroft7 suggested an alternative method of searching for high-TC superconductors that uses other elements, metals or nonmetals, to precompress the hydrogen atoms, which should lead to a dramatic decrease in the metallization pressure. A decade later this idea found its experimental proof. Extraordinarily high superconducting transition temperatures were demonstrated in compressed \(Im\bar 3m\)-H3S8,9,10,11 (203 K at 150 GPa), \(Im\bar 3m\)-YH612 and P63/mmc-YH913 (224 K at 166 GPa and 243 K at 237 GPa, respectively), \(Fm\bar 3m\)-ThH1014 (161 K at 174 GPa), P63/mmc-CeH9 (~100 K)15, and lanthanum decahydride \(Fm\bar 3m\)-LaH1016,17,18 with TC > 250 K at 175 GPa.
The neighbor of lanthanum, barium is a promising element for superhydride synthesis. The calculated maximum TC is only about 30–38 K19,20 for predicted P4/mmm-BaH6 stable at 100 GPa, which has a hydrogen sublattice consisting of H2 molecules and H– anions19. Lower barium hydride, BaH2, well-known for its extraordinarily anionic (H–) conductivity21, exists in Pnma modification below 2.3 GPa, whereas above 2.3 GPa it undergoes a transition to hexagonal Ni2In-type P63/mmc phase22. At pressures above 41 GPa, BaH2 transforms into P6/mmm modification, which metallizes at over 44 GPa, but its superconducting TC is close to zero23. So far, no relevant experiments at pressures above 50 GPa have been reported.
In this work we experimentally and theoretically investigate the chemistry of the barium-hydrogen system at pressures from 75 to 173 GPa filling the gap of previous studies. We discover new pseudocubic BaH12 that has molecular structure with H2 and H3– molecular units and detached H12 chains formed due to Peierls-type distortion. These structural features lead to metallic conductivity of unique molecular hydride and to the superconducting transition around 20 K at 140 GPa.
Results
Synthesis at 160 GPa and Stability of BaH12
To investigate the formation of new chemical compounds in the Ba–H system at high pressures, we loaded four high-pressure diamond anvil cells (DACs #B0-B3) with sublimated ammonia borane NH3BH3 (AB), used as both a source of hydrogen and a pressure transmitting medium. A tungsten foil with a thickness of about 20 μm was used as a gasket. Additional parameters of the high-pressure diamond anvil cells are given in Supplementary Table S1.
The first attempt of the experimental synthesis was made in DAC #B1 heated to 1700 K by an infrared laser pulse with a duration of ~0.5 s at a pressure of 160 GPa. During heating, the Ba particle underwent significant expansion and remained nontransparent. The obtained synchrotron X-ray diffraction pattern (XRD, λ = 0.62 Å, Fig. 1a) consists of a series of strong reflections specific to cubic crystals. Decreasing the pressure in DAC #B1 to 119 GPa (Fig. 1b) gave a series of diffraction patterns that can mostly be indexed by a slightly distorted face-centered cubic structure (e.g., pseudocubic Cmc21, Fig. 1a). Recently, similar cubic diffraction patterns have been observed at pressures above 150 GPa for the La–H (fcc-LaH10)17,18 and Th-H (fcc-ThH10)14 systems. By analogy with the La–H system, and considering the lack of previously predicted cubic superhydrides BaHx19,20,21, we used the USPEX code24,25,26 to perform theoretical crystal structure evolutionary searches, both variable- and fixed-composition, for stable Ba–H compounds at pressures of 100–200 GPa and temperatures of 0–2000 K.
a Experimental X-ray diffraction pattern from DAC #B1 at 160 GPa and the Le Bail refinement of the pseudocubic Cmc21-BaH12 phase. The experimental data, fitted line, and residues are shown in red, black, and green, respectively. Unidentified reflections are indicated by asterisks. b X-ray diffraction patterns at pressures of 119 to 160 GPa. The inset shows the projection of the Cmc21 structure to the (ac) plane. The hydrogen network is shown by light blue lines. c Convex hulls of the Ba–H system at 100, 150, and 200 GPa calculated with zero-point energy (ZPE) contribution. d Calculated equations of state for different possible crystal modifications of BaH12 (fcc, I4/mmm, and Cmc21) and Ba+nH2. The experimental data are shown by hollow squares.
According to the USPEX calculations, P6/mmm-BaH2 remains stable up to 150–200 GPa (Fig. 1c; Supplementary Tables S7–S12, Supplementary Figs. S2 and S3). This compound was experimentally detected in DAC #B0 at 173–130 GPa with the cell volume ~3% smaller than theoretically predicted (Supplementary Table S14). At 100–200 GPa, several new barium polyhydrides lying on or near the convex hulls were found: BaH6, BaH10, and BaH12 with the unit cell Ba4H48 and Ba8H96 (Fig. 1c). In subsequent experiments at 142 and 154–173 GPa we have detected a series of reflections that can be indexed by BaH6 and BaH10 with the unit cell volumes close to the calculated ones (see Supporting Information, p. S25-27). However, the main phase in almost all diffraction patterns is the pseudocubic barium superhydride which will be described below.
The analysis of the experimental data within space group \(Fm\bar 3m\) (Fig. 1b and Supplementary Table S3) of Ba-sublattice and its comparison with density functional theory (DFT) calculations show that the stoichiometry of barium hydride synthesized in DAC #B1 is close to BaH12. Examining the results of the fixed-composition search, we found that an ideal \(Fm\bar 3m\)-BaH12 (similar to fcc-YB12) is unstable and cannot exist, while pseudocubic P21-BaH12, whose predicted diffraction pattern is similar to the experimental one, lies on the convex hull at 100–150 GPa. There are also pseudocubic P1-Ba8H96, located very close to the convex hull at 150 GPa, and Cmc21-BaH12 (= Ba4H48) with a similar X-ray diffraction (XRD) pattern, lying a bit farther. Above 190 GPa the P21-BaH12 transforms to other possible candidate, orthorhombic Immm-BaH12, which stabilizes between 150 and 200 GPa, but does not correspond to the experimental XRD pattern (Fig. 1a, Supplementary Fig. S1) and is not considered further.
The computed equation of state of \(Fm\bar 3m\)-BaH12 (Fig. 1d) corresponds well to the experimental volume-pressure dependence above 100 GPa. However, the DFT calculations show that the ideal \(Fm\bar 3m\) barium sublattice is unstable (it is > 0.19 eV/atom above the convex hull, Supplementary Fig. S4) both thermodynamically and dynamically, and transforms spontaneously to Cmc21 or P21 via distortion (Fig. 2). Studying the temperature dependence of the Gibbs free energy (Fig. 2a), we found that P21-BaH12 is the most stable modification at 0–2000 K and 100–150 GPa. Moreover, high-symmetry cubic phases cannot explain the weak reflections at 8.9–9.4°, 14.5, 16, 19.5, and 20.6° present in many XRD patterns (Fig. 1a, b).
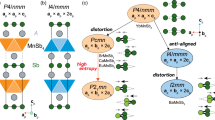
a Dependence of the Gibbs free energies of formation on the temperature for different modifications of BaH12 — Immm, pseudocubic Cmc21, P21, and P1 — calculated within the harmonic approach in the temperature range of 100 to 2000 K at 100, 150, and 200 GPa. b Phonon and c electron densities of states for pseudocubic BaH12 structures with various degrees of distortion: Cmc21, P21, and P1.
To clarify the question of dynamical stability of pseudocubic structures, we calculated a series of phonon densities of states for different modifications of BaH12 (Fig. 2b). Within the harmonic approach, symmetric and corresponding to the experimental data Cmc21-BaH12 has a number of imaginary phonon modes. Its distortion to much more stable P21-BaH12 leads to the disappearance of many of the imaginary phonon modes and deepening of the pseudogap (Fig. 2c) in the electronic density of states N(E). The subsequent distortion of P21 to P1 converts BaH12 to a semiconductor with a bandgap exceeding 0.5 eV. However, the experimental data show that BaH12 remains opaque in the visible range, does not give Raman signals (Supplementary Figs. S39-40), retains an almost fcc crystal structure, and exhibits metallic properties (see next sections) down to 75 GPa. For this reason, the electronic band structure and parameters of the superconducting state were further investigated only for Cmc21-BaH12, which does not have a bandgap at 100–150 GPa. Stability of all considered polymorphic modifications of BaH12 at different pressures with respect to other Ba-H phases and with respect to each other are shown in Supplementary Figs. S4 and S5 (see Supporting Information).
The comparative analysis of Cmc21, P21, and P1 structures of BaH12 shows that semimetallic Cmc21 explains well the experimental results of X-ray diffraction (see Supplementary Fig. S1, Supporting Information) and lies closer to the convex hull than \(Fm\bar 3m\) or I4/mmm modifications. P1-BaH12 shows a complex picture of splitting of the diffraction signals, both P1-BaH12 and P21-BaH12 have a bandgap above 0.5 eV at 100 GPa (Supplementary Fig. S38b) which does not correspond to the experimental data. Therefore, pseudocubic Cmc21-BaH12, whose cell volume is near that of the close-packed \(Fm\bar 3m\)-BaH12, is the appropriate explanation of the experimental results despite the presence of a few imaginary phonon modes.
The molecular dynamics simulation of Cmc21-BaH12 and P21-BaH12 at 10–1500 K, after averaging the coordinates, both lead to a distorted pseudocubic P1-BaH12 with the similar XRD pattern. However, all structures retrieved by molecular dynamics are less stable both dynamically and thermodynamically than P1-BaH12, P21-BaH12, and Cmc21-BaH12 found by USPEX. More accurate analysis accounting for the anharmonic nature of hydrogen oscillations27, which is actually beyond the scope of this work, may help to explain the experimental stability of higher-symmetry BaH12 modifications compared to lower-symmetry P1-BaH12.
Synthesis of BaH12 at 146 GPa
Similar X-ray diffraction patterns were obtained in the next experiment (DAC #B2) where the Ba sample was heated at an initial pressure of 146 GPa, which led to a decrease in pressure to 140 GPa. During the heating and subsequent unloading of the cell, the sample remained opaque down to ~40 GPa. Unlike the synthesis at high pressure (cell #B1, 160 GPa, Fig. 1a, b), in this experiment we observed many more side phases and corresponding side reflections than before (Fig. 3 and Supporting Information).
a Le Bail refinement of pseudocubic Cmc21-BaH12 and bcc-W at 93 GPa. The experimental data, fitted line, and residues are shown in red, black, and green, respectively. Inset shows the 2D diffraction image. Weak reflections, indicated by asterisks, may correspond to the impurity: possibly P63/mmc or P63mc-BaH12. b, c Microphotographs of the culet of cell #B2 with the Ba/AB sample before and after the laser heating. d Experimental XRD patterns from cell #B2 at pressures decreasing from 138 to 111 GPa. e, f, g Powder X-ray diffraction images at 128, 111, and 75 GPa. Reflections corresponding to the crystallographic planes in the ideal \(Fm\bar 3m\)-BaH12 phase are indicated by arrows.
Similar to the experiment with DAC #B1, five reflections from the pseudocubic Ba sublattice dominate in a wide range of pressures (65–140 GPa), whereas side reflections change their intensities and, at some pressures, almost disappear (Fig. 3d and Supplementary Figs. S33 and S35). The diffraction circles corresponding to the ideal cubic barium sublattice have pronounced granularity (Fig. 3e–g, Supplementary Fig. S34), which suggests that all “cubic” reflections belong to the same phase.
At pressures below 65 GPa, it is no longer possible to refine the cell parameters of pseudocubic BaH12. The parameters of the Cmc21-BaH12 unit cell, refined to the experimental data, are presented in Supplementary Table S6. Fitting this pressure-volume data in the pressure range from 75 to 173 GPa by the third-order Birch–Murnaghan equation of state28 gives the cell volume V100 = 45.47 ± 0.13 Å3, bulk modulus K100 = 305 ± 8.5 GPa, and its derivative with respect to pressure \(K_{100}^\prime\) = 3.8 ± 0.48 (the index 100 designates values at 100 GPa). Fitting the theoretical data yields similar values: V100 = 46.0 Å3, K100 = 315.9 GPa, and \(K_{100}^\prime\) = 2.94.
Synthesis of BaH12 at 90 GPa
In the experiment with DAC #B3, we investigated the possibility to synthesize BaH12 at pressures below 100 GPa. After the laser heating of Ba/AB to 1600 K, the pressure in the cell decreased from 90 to 84 GPa. The observed diffraction pattern is generally similar to those in the previous experiments with DAC #B1, except the presence of the impurity, h-BaH~12, whose reflections may be indexed by hexagonal space groups P63/mmc or P63mc (a = 3.955(7) Å, c = 7.650(7) Å, V = 51.84 Å3 at 78 GPa). For the main set of reflections, slightly distorted cubic BaH12 is the best solution (Fig. 4). The refined cell parameters of BaH12 (Supplementary Table S4) agree well with the results obtained previously with DACs #B1 and B2. When the pressure was reduced to 78 GPa, barium dodecahydride began to decompose, and subsequent diffraction patterns (e.g., at 68 GPa, see Supporting Information) show a complex image of broad reflections that confirms the lower experimental bound of BaH12 stability of ~75 GPa mentioned above.
Loaded Ba/AB sample a before and b after the laser heating in DAC #B3. Experimental XRD pattern and the Le Bail refinement of c the ideal \(Fm\bar 3m\)-BaH12 structure at 84 GPa and d Cmc21-BaH12 obtained via the distortion of \(Fm\bar 3m\)-BaH12. The reflections indicated by asterisks may correspond to unidentified hexagonal barium polyhydride BaH~12. The experimental data, model fit for the structure, and residues are shown in red, black, and green, respectively. e Electron localization function (ELF), projected onto f the (100) plane and g the \((11\bar 1)\) plane, and Bader charges of the Ba and H atoms in Cmc21-BaH12 at 150 GPa. h Contribution of barium and hydrogen to the electronic density of states of BaH12. i d-character of Ba electrons near the Fermi level.
Discussion
Electronic properties of BaH12
BaH12 is the first known metal hydride with such a high hydrogen content that is stable at such low pressures (~75 GPa). We further investigated its electronic structure and the charge state of the hydrogen and barium atoms. The electron localization function (ELF) analysis29 (Fig. 4e–g) shows that hydrogen in BaH12, similar to NaH730, is present in the form of H2 (dH–H = 0.78 Å) and almost linear H3 (dH–H = 0.81 and 1.07 Å) molecular fragments that form separate flat horseshoe-like H12 chains (dH–H < 1.27 Å, Fig. 4).
Bader charge analysis of Cmc21-BaH12, performed in accordance with our previous experience31,32 (Supplementary Table S18), shows that the Ba atoms serve as a source of electrons for the hydrogen sublattice. The charge of the barium atoms in BaH12 is +1.15 at 150 GPa, whereas most of the hydrogen atoms have a negative charge. In the H3 fragments, the charge of the end atoms is close to –0.2 and –0.27, while the H bridge has a small positive charge of +0.06 (Fig. 4e–g). In general, H3– anion, similar to one found in the structure of NaH730, has a total charge of –0.4 | e | , whereas molecular fragments H2 (dH–H = 0.78 Å) have a charge of only –0.1 | e | . Therefore, the Ba–H bonds in BaH12 have substantial ionic character, whereas the H–H bonds are mainly covalent.
The low electronic density of states N(E) in semimetallic Cmc21-BaH12 looks typical for one-dimensional …H–H–H… chains (Fig. 4h, Supplementary Figs. S36 and S38) which are divided into H2, H3 fragments due to the Peierls-type distortion33. In fact, all of the discussed structures of BaH12 can be viewed as a result of Peierls-type distortion. The main contribution to N(EF), 83% at 150 GPa, comes from hydrogen (Fig. 4h), and ¾ of this contribution is related to s orbitals. At 150 GPa, barium in BaH12 exhibits the properties of a d-block element, and its bonding orbitals have a significant d-character (Fig. 4i). Electrical conductivity is localized in the H layers consisting of quasi-one-dimensional …H–H–H… chains which are interconnected in non-trivial way (Fig. 4e–g, Supplementary Table S2 for crystal structure). Thus, barium dodecahydride is the first known molecular superhydride with metallic conductivity embedded in layers and one-dimensional chains of molecular hydrogen.
Superconductivity of BaH12
On the basis of powder diffraction experiments and thermodynamic calculations alone, we cannot unambiguously determine the structure of the H sublattice in BaH12, which is essential for understanding superconductivity. To clarify this, we measured the electrical resistance R of barium hydride samples using the well-known four-probe technique in the temperature range of 2–300 K. At pressures of 90–140 GPa, all five BaHx samples (DACs E#1-5, see Supporting Information) synthesized in electric DACs behave as typical metals with an almost linear decrease of R(T). At low temperatures the resistance of the samples drops sharply, indicating a possible superconducting transition at about 5–7 K below 130 GPa (Supplementary Fig. S41), and ~20 K at 140 GPa (cell #E5, Fig. 5a). This DAC #E5 was assembled with an 80 µm diamond anvil culet, c-BN/epoxy insulating gasket, 45 × 32 µm Ba piece, and sputtered 0.5 µm thick Mo electrodes. After the laser heating at 1600 K and 140 GPa, the Ba/AB sample demonstrated the superconducting transition at around 20 K (Fig. 5a). When we tried to change the pressure, the cell collapsed and pressure dropped to 65 GPa.
a Four-probe measurements of the electrical resistance R(T) of the BaHx sample synthesized using the laser heating at 140 GPa. The superconducting transition was detected at ~20 K. b Calculated Eliashberg spectral functions and electron-phonon coupling parameter (λ) for pseudocubic BaH12 at 140 (black) and 150 GPa (blue).
The obtained data together with the measured Raman spectra and optical microscopy exclude low-symmetry BaH12 semiconducting structures, leaving for consideration only metallic and semimetallic modifications (Supplementary Figs. S39 and S40).
The harmonic DFT calculations (Fig. 5b) demonstrate that the low density of electronic states near the Fermi level in Cmc21-BaH12 is associated with a weak electron-phonon coupling, mostly related to low-frequency Ba and H phonon modes (1–10 THz), resulting in relatively low λ = 1.02, ωlog = 677 K, TC = 39–53 K, and µ0Hc2(0) = 5.1–7 T at 150 GPa (µ* = 0.15–0.1). Decreasing pressure leads to a decrease of λ and TC (from 53 to 46 K) at 140 GPa with a slope dTC/dP = 0.7 K/GPa.
One of the roles of metal atoms in superhydrides is to donate electrons to antibonding orbitals of the H2 molecules and weaken the H-H bonds. In BaH12, each H atom accepts few electrons, on average 0.16 electrons. As a result, H2 and H3 groups are still present in the structure, and we have a rather low TС. We think that at high pressures, due to dissociation of molecular groups, BaH12 may have a network of weak H-H bonds (rather than discrete H2 and H3-groups) and, as a result, a much higher TС. Increasing the pressure will also facilitate further metallization of BaH12 and symmetrization of the hydrogen sublattice, increasing N(EF). To estimate the possible improvement, we calculated at 120-135 GPa the superconducting parameters of I4/mmm-BaH12 and \(Fm\bar 3m\)-BaH12, isostructural to YB12, the structures that were considered as possible solutions at the first step of the XRD interpretation. The calculations show that filling of the pseudogap in N(E) makes it possible to reach TC ~ 200 K with λ ≥ 3 in these compounds (Supplementary Figs. S42 and S43).
In conclusion, studying the high-pressure chemistry of the Ba–H system in four independent DACs we successfully synthesized novel barium superhydride BaH12 with a pseudocubic crystal structure, stabilized in the pressure range of 75–173 GPa. The compound was obtained by laser-heating metallic barium with an excess of ammonia borane compressed to 173, 160, 146, and 90 GPa. The Ba sublattice structure of BaH12 was resolved using the synchrotron XRD, evolutionary structure prediction, and several postprocessing Python scripts, including an XRD matching algorithm. Discovered BaH12 has unique metallic conductivity, localized in the layers of molecular hydrogen, and the highest hydrogen content (>92 mol%) among all metal hydrides synthesized so far. The experimentally established lower limit of stability of barium dodecahydride is 75 GPa. The third-order Birch–Murnaghan equation of state and unit cell parameters of BaH12 were found in the pressure range of 75–173 GPa: V100 = 45.47 ± 0.13 Å3, K100 = 305 ± 8.5 GPa, and \(K_{100}^\prime\) = 3.8 ± 0.48. The ab initio calculations confirm a small distortion of the ideal fcc-barium sublattice to space group Cmc21 or P21, determined by the presence of additional weak reflections in the diffraction patterns. The impurity phase analysis indicates possible presence of BaH6 and BaH10. According to the theoretical calculations and experimental measurements, BaH12 exhibits metallic and superconducting properties, with TC = 20 K at 140 GPa, and its crystal structure contains H2 and H3– groups. The results of these experiments confirm that the comparative stability of superhydrides increases with the increase of the period number of a hydride-forming element in the periodic table20. Our work opens prospects for the synthesis of even more hydrogen rich compounds like predicted LaH1634 and ErH1520, and new ternary high-TC polyhydrides in such systems as Ba-Y-H and Ba-La-H.
Methods
Experimental details
The barium metal samples with a purity of 99.99% were purchased from Alfa Aesar. All diamond anvil cells (100 μm and 50 μm culets) were loaded with a metallic Ba sample and sublimated ammonia borane (AB) in an argon glove box. The tungsten gasket had a thickness of 20 ± 2 μm. The heating was carried out by 2–3 pulses of an infrared laser (1.07 μm, Nd:YAG), each pulse had a duration of 0.3–0.5 s. The temperature was determined using the decay of blackbody radiation within the Planck formula. The applied pressure was measured by the edge of diamond Raman signal35 using the Horiba LabRAM HR800 Ev spectrometer with an exposure time of 10 s. The XRD patterns from samples in diamond anvil cells (DACs) were recorded on the BL15U1 synchrotron beamline at the Shanghai Synchrotron Research Facility (SSRF, China) using a focused (5 × 12 μm) monochromatic X-ray beam with a linear polarization (20 keV, 0.6199 Å). Mar165 CCD was used as a detector.
The experiment with DAC #B0 was carried out at the Advanced Photon Source, Argonne, U.S. The loaded particle was successively heated up to 2100 K using millisecond-long pulses (4 × 0.04 s) of a 1064 nm Yb-doped fiber laser. We used this pulsed laser heating mode to avoid the premature breakage of a diamond. The synchrotron XRD measurements (the X-ray wavelength was 0.2952 Å) were performed at the GSECARS of the Advanced Photon Source36 with about 3 × 4 µm X-ray beam spot.
The experimental XRD images were analyzed and integrated using Dioptas software package (version 0.5)37. The full profile analysis of the diffraction patterns and the calculation of the unit cell parameters were performed in the Materials studio38 and JANA200639 using the Le Bail method40.
To investigate the electrical resistivity of barium polyhydrides, we performed 5 runs of measurements in Cu-Be DACs #E1-5 using the four-probe technique. The preparation of all cells was similar. Tungsten gasket with initial thickness of 250 μm was precompressed to about 25 GPa. Then a hole with a diameter of 20% bigger than the culet diameter was drilled using pulse laser (532 nm). Cubic boron nitride (c-BN) powder mixed with epoxy was used as an insulating layer. We filled the chamber with MgO and compressed it to about 5 GPa. Then, in the obtained transparent MgO layer, a hole with diameter about 40μm was drilled by laser. UV lithography was used to prepare four electrodes on the diamond culet. We deposited the 500 nm thick Mo layer by magnetron sputtering (field of 200 V at 300 K) and removed the excess of metal by acid etching. Four deposited Mo electrodes were extended by platinum foil. The chamber was filled with the sublimated ammonia borane (AB) and a small piece of Ba was placed on the culet of upper diamond with the four electrodes. All preparations were made in an argon glove box (O2 < 0.1 ppm, H2O < 0.01 ppm). After that, the DACs were closed and compressed to a required pressure. We used 1.07 µm infrared pulse (~0.1 s, 1600 K) laser to heat the Ba/AB samples. Electrical resistance of the samples was studied in a cryostat (1.5-300 K, JANIS Research Company Inc.; in magnetic fields 0-9 T, Cryomagnetics Inc.) with applied current of 1 mA. More details about DACs #E1-5 are given in the Table S20.
Computational details
The study is based on the structural search for stable compounds in the Ba–H system using the USPEX code, for pressures of 50, 100, 150, 200, and 300 GPa, with a variable-composition evolutionary search from 0 to 24 atoms of each type (Ba, H). The first generation of the search (120 structures) was created using a random symmetric generator, all subsequent generations (100 structures) contained 20% of random structures and 80% of those created using the heredity, soft mutation, and transmutation operators. The results contain the files extended_convex_hull and extended_convex_hull_POSCARS, which were postprocessed using the Python scripts change_pressure.py, split_CIFs.py and xr_screening.py (see Scripts for XRD Postprocessing with USPEX section). The postprocessing script change_pressure.py performs an isotropic deformation of the unit cell of structures predicted by USPEX, bringing them to approximately the experimental pressure. All three lattice constants of the structures are multiplied by a factor k, calculated under the assumption of validity of the Birch–Murnaghan equation of state28 with the bulk modulus K0 = 300 GPa and its first derivative K’ = 3. This approach is a quick alternative to the script that uses a crude DFT reoptimization of a set of theoretically possible structures, bringing them to the experimental pressure. The script split_CIFs.py converts the set of POSCARS recorded in the extended_convex_hull_POSCARS file into a set of CIF files, simultaneously symmetrizing the unit cells and sorting the files by ascending fitness (the distance from the convex hull). The CIF files created in such a way can be directly analyzed using Dioptas37, JANA200639 and other software. Finally, the script xr_screening.py automatically searches for the structures found by USPEX and translated to the experimental pressure that exhibit a high similarity between the experimental and predicted XRD patterns (the latter are obtained using pymatgen41 Python library). The analysis of complex mixtures consisted of two steps: first, we searched for the main component having the most intense reflections, then the already explained reflections were excluded to analyze the side phases.
To calculate the equations of state (EoS) of BaH12, we performed structure relaxations of phases at various pressures using the density functional theory (DFT)42,43 within the generalized gradient approximation (Perdew–Burke–Ernzerhof functional)44 and the projector augmented wave method45,46,47,48,49 as implemented in the VASP code15,16,17. The plane wave kinetic energy cutoff was set to 1000 eV, and the Brillouin zone was sampled using the Γ-centered k-points meshes with a resolution of 2π × 0.05 Å−1. The obtained dependences of the unit cell volume on the pressure were fitted by the Birch–Murnaghan equation28 to determine the main parameters of the EoS — the equilibrium volume V0, bulk modulus K0, and its derivative with respect to pressure K’ — using EOSfit7 software50. We also calculated the phonon densities of states for the studied materials using the finite displacement method (VASP and PHONOPY)51,52.
The calculations of the phonons, electron-phonon coupling, and superconducting TC were carried out with QUANTUM ESPRESSO (QE) package53,54 using the density functional perturbation theory55, employing the plane-wave generalized gradient approximation with Perdew–Burke–Ernzerhof functional44. In our ab initio calculations of the electron-phonon coupling (EPC) parameter λ of Cmc21-Ba4H48, the first Brillouin zone was sampled by 2 × 2 × 2 q-points mesh and 4 × 4 × 4 or 8 × 8 × 8 k-points meshes with the smearing σ = 0.005–0.05 Ry that approximates the zero-width limits in the calculation of λ. The critical temperature TC was calculated using the Allen–Dynes equations56.
Bader charges were calculated using Critic257,58 software with the atomic partition generated using the YT59 method. The electron localization function (ELF) of BaH12 and isosurface are shown for an isovalue of 0.12. The lattice planes (100) and \(\left( {11\bar 1} \right)\) are shown at distances from the origin of 0 and 2.289 Å, respectively. We projected the ELF on these planes to show H3 and H2 bonding.
The average structure of BaH12 was analyzed using the ab initio molecular dynamics (AIMD) simulations within the general gradient approximation44 and using the augmented plane wave method45,47,48,49 implemented in VASP software30,31,32. The total number of atoms in the model was 52, including 48 hydrogen atoms and 4 barium atoms (Cmc21-Ba4H48). The positions of the barium atoms were fixed during the simulation. The energy cutoff was set to 400 eV. The behavior of the hydrogen atoms in the BaH12 crystal structure was studied upon annealing from 1500 to 10 K using the Nosé–Hoover thermostat60,61. The total simulation time was 10 ps with the time step of 1 fs.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files.
References
Oganov, A. R., Pickard, C. J., Zhu, Q. & Needs, R. J. Structure prediction drives materials discovery. Nat. Rev. Mater. 4, 331–348 (2019).
Wigner, E. & Huntington, H. B. On the possibility of a metallic modification of hydrogen. J. Chem. Phys. 3, 764–770 (1935).
McMahon, J. M., Morales, M. A., Pierleoni, C. & Ceperley, D. M. The properties of hydrogen and helium under extreme conditions. Rev. Mod. Phys. 84, 1607–1653 (2012).
Azadi, S., Monserrat, B., Foulkes, W. M. C. & Needs, R. J. Dissociation of high-pressure solid molecular hydrogen: a quantum Monte Carlo and anharmonic vibrational study. Phys. Rev. Lett. 112, 165501 (2014).
McMinis, J., Clay, R. C., Lee, D. & Morales, M. A. Molecular to atomic phase transition in hydrogen under high pressure. Phys. Rev. Lett. 114, 105305 (2015).
Eremets, M. I., Drozdov, A. P., Kong, P. P. & Wang, H. Semimetallic molecular hydrogen at pressure above 350 GPa. Nat. Phys. 15, 1246–1249 (2019).
Ashcroft, N. W. Hydrogen dominant metallic alloys: high temperature superconductors? Phys. Rev. Lett. 92, 187002 (2004).
Duan, D. et al. Pressure-induced metallization of dense (H2S)2H2 with high-Tc superconductivity. Sci. Rep. 4, 6968 (2014).
Drozdov, A. P., Eremets, M. I., Troyan, I. A., Ksenofontov, V. & Shylin, S. I. Conventional superconductivity at 203 kelvin at high pressures in the sulfur hydride system. Nature 525, 73–76 (2015).
Troyan, I. et al. Observation of superconductivity in hydrogen sulfide from nuclear resonant scattering. Science 351, 1303–1306 (2016).
Huang, X. et al. High-temperature superconductivity in sulfur hydride evidenced by alternating-current magnetic susceptibility. Natl Sci. Rev. 6, 713–718 (2019).
Troyan, I. A. et al. Anomalous high-temperature superconductivity in YH6. arXiv:1908.01534 (2020).
Kong, P. P. et al. Superconductivity up to 243 K in yttrium hydrides under high pressure. Preprint at https://arxiv.org/abs/1909.10482 (2019).
Semenok, D. V. et al. Superconductivity at 161K in thorium hydride ThH10: synthesis and properties. Mater. Today 33, 36–44 (2020).
Li, X. et al. Polyhydride CeH9 with an atomic-like hydrogen clathrate structure. Nat. Commun. 10, 3461 (2019).
Liu, H., Naumov, I. I., Hoffmann, R., Ashcroft, N. W. & Hemley, R. J. Potential high-Tc superconducting lanthanum and yttrium hydrides at high pressure. Proc. Natl Acad. Sci. USA 114, 6990–6995 (2017).
Drozdov, A. P. et al. Superconductivity at 250 K in lanthanum hydride under high pressures. Nature 569, 528 (2019).
Somayazulu, M. et al. Evidence for superconductivity above 260 K in lanthanum superhydride at megabar pressures. Phys. Rev. Lett. 122, 027001 (2019).
Hooper, J., Altintas, B., Shamp, A. & Zurek, E. Polyhydrides of the alkaline earth metals: a look at the extremes under pressure. J. Phys. Chem. C. 117, 2982–2992 (2013).
Semenok, D. V., Kruglov, I. A., Kvashnin, A. G. & Oganov, A. R. On distribution of superconductivity in metal hydrides. Curr. Opin. Solid State Mater. Sci. 7, 100808 (2020).
Verbraeken, M. C., Cheung, C., Suard, E. & Irvine, J. T. S. High H − ionic conductivity in barium hydride. Nat. Mater. 14, 95–100 (2015).
Zhang, X. et al. Hydride ion (H−) transport behavior in barium hydride under high pressure. Phys. Chem. Chem. Phys. 20, 8917–8923 (2018).
Tse, J. S. et al. Structure and electronic properties of BaH2 at high pressure. Solid State Commun. 149, 1944–1946 (2009).
Oganov, A. R. & Glass, C. W. Crystal structure prediction using ab initio evolutionary techniques: Principles and applications. J. Chem. Phys. 124, 244704 (2006).
Oganov, A. R., Lyakhov, A. O. & Valle, M. How evolutionary crystal structure prediction works—and why. Acc. Chem. Res. 44, 227–237 (2011).
Lyakhov, A. O., Oganov, A. R., Stokes, H. T. & Zhu, Q. New developments in evolutionary structure prediction algorithm USPEX. Comput. Phys. Commun. 184, 1172–1182 (2013).
Errea, I. et al. Quantum crystal structure in the 250-kelvin superconducting lanthanum hydride. Nature 578, 66–69 (2020).
Birch, F. Finite elastic strain of cubic crystals. Phys. Rev. 71, 809–824 (1947).
Savin, A., Nesper, R., Wengert, S. & Fässler, T. F. ELF: the electron localization function. Angew. Chem. Int. Ed. Engl. 36, 1808–1832 (1997).
Struzhkin, V. V. et al. Synthesis of sodium polyhydrides at high pressures. Nat. Commun. 7, 12267 (2016).
Tantardini, C. & Benassi, E. Crystal structure resolution of an insulator due to the cooperative Jahn–Teller effect through Bader’s theory: the challenging case of cobaltite oxide Y114. Dalton Trans. 47, 5483–5491 (2018).
Tantardini, C. & Benassi, E. Topology vs. thermodynamics in chemical reactions: the instability of PH 5. Phys. Chem. Chem. Phys. 19, 27779–27785 (2017).
Peierls, R. E. Quantum Theory of Solids. Quantum Theory of Solids (Oxford University Press).
Kruglov, I. A. et al. Superconductivity of LaH10 and LaH16 polyhydrides. Phys. Rev. B 101, 024508 (2020).
Akahama, Y. & Kawamura, H. Pressure calibration of diamond anvil Raman gauge to 410 GPa. J. Phys. Conf. Ser. 215, 012195 (2010).
Prakapenka, V. B. et al. Advanced flat top laser heating system for high pressure research at GSECARS: application to the melting behavior of germanium. High Press. Res. 28, 225–235 (2008).
Prescher, C. & Prakapenka, V. B. DIOPTAS: a program for reduction of two-dimensional X-ray diffraction data and data exploration. High. Press. Res. 35, 223–230 (2015).
Paufler, P. R. A. Young (ed.). The Rietveld Method. International Union of Crystallography. Oxford University Press 1993. 298 p. Price £ 45.00. ISBN 0–19–855577–6. Cryst. Res. Technol. 30, 494–494 (1995).
Petříček, V., Dušek, M. & Palatinus, L. Crystallographic computing system JANA2006: general features. Z. F.ür. Krist. - Cryst. Mater. 229, 345–352 (2014).
Bail, A. L. Whole powder pattern decomposition methods and applications: a retrospection. Powder Diffr. 20, 316–326 (2005).
Ong, S. P. et al. Python Materials Genomics (pymatgen): a robust, open-source python library for materials analysis. Comp. Mat. Sci. 68, 314–319 (2013).
Hohenberg, P. & Kohn, W. Inhomogeneous electron gas. Phys. Rev. 136, B864–B871 (1964).
Kohn, W. & Sham, L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133–A1138 (1965).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Angel, R. J., Alvaro, M. & Gonzalez-Platas, J. EosFit7c and a Fortran module (library) for equation of state calculations. Z. F.ür. Krist. - Cryst. Mater. 229, 405–419 (2014).
Togo, A. & Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 108, 1–5 (2015).
Togo, A., Oba, F. & Tanaka, I. First-principles calculations of the ferroelastic transition between rutile-type and CaCl2-type SiO2 at high pressures. Phys. Rev. B 78, 134106 (2008).
Giannozzi, P. et al. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 21, 395502 (2009).
Giannozzi, P. et al. Advanced capabilities for materials modelling with quantum ESPRESSO. J. Phys. Condens. Matter 29, 465901 (2017).
Baroni, S., de Gironcoli, S., Dal Corso, A. & Giannozzi, P. Phonons and related crystal properties from density-functional perturbation theory. Rev. Mod. Phys. 73, 515–562 (2001).
Allen, P. B. & Dynes, R. C. Transition temperature of strong-coupled superconductors reanalyzed. Phys. Rev. B 12, 905–922 (1975).
Otero-de-la-Roza, A., Blanco, M. A., Pendás, A. M. & Luaña, V. Critic: a new program for the topological analysis of solid-state electron densities. Comput. Phys. Commun. 180, 157–166 (2009).
Otero-de-la-Roza, A., Johnson, E. R. & Luaña, V. Critic2: A program for real-space analysis of quantum chemical interactions in solids. Comput. Phys. Commun. 185, 1007–1018 (2014).
Yu, M. & Trinkle, D. R. Accurate and efficient algorithm for Bader charge integration. J. Chem. Phys. 134, 064111 (2011).
Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 81, 511–519 (1984).
Hoover, W. G. Canonical dynamics: equilibrium phase-space distributions. Phys. Rev. A 31, 1695–1697 (1985).
Acknowledgements
The authors thank the staff of the Shanghai Synchrotron Radiation Facility and express their gratitude to Bingbing Liu’s group (Jilin University) for their help in the laser heating of samples. This work was supported by the National Key R&D Program of China (Grant No. 2018YFA0305900), the National Natural Science Foundation of China (Grant Nos. 51632002, 11974133 and 51720105007), the Program for Changjiang Scholars and Innovative Research Team in University (Grant No. IRT_15R23). Work of D.V.S. and A.G.K. is supported by Russian Science Foundation (Grant No. 19-72-30043); work of A.R.O. is funded by Russian Ministry of Science and Technology (Grant No. 2711.2020.2 to leading scientific schools). Portions of this work were performed at GeoSoilEnviroCARS (The University of Chicago, Sector 13), Advanced Photon Source (APS), Argonne National Laboratory. GeoSoilEnviroCARS is supported by the National Science Foundation — Earth Sciences (EAR — 1634415) and Department of Energy — GeoSciences (DE-FG02-94ER14466). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. A.G.K., D.V.S. thank the Russian Foundation for Basic Research (Grant No. 19-03-00100). The reported study was funded by RFBR, project 20-32-90099. We thank the Russian Ministry of Science and Higher Education agreement No. 075-15-2020-808. A.F.G. acknowledges the support of the Army Research Office. We thank Dr. Christian Tantardini (Skoltech) for help with the calculation of the Bader charges, Dr. Di Zhou (JLU) for preparing the convex hulls and α2F(ω) diagrams, and Igor Grishin (Skoltech) for proofreading of the manuscript.
Author information
Authors and Affiliations
Contributions
X.H., W.C., D.V.S., A.G.K., A.R.O. and T.C. conceived this project. W.C., D.V.S., X.H. performed the experiment, D.V.S., A.G.K, I.A.K., H.S., D.D., A.R.O., M.G. and T.C. prepared the theoretical calculations and analysis. W.C., X.H., A.F.G. and V.B.P. performed X-ray measurements of synthesized samples. W.C., D.V.S., A.G.K., A.R.O., X.H. and T.C. wrote and revised the paper. All the authors discussed the results and offered useful inputs.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Commuications thanks Jun-Hyung Cho and other, anonymous, reviewers for their contributions to the peer review of this work. Peer review reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, W., Semenok, D.V., Kvashnin, A.G. et al. Synthesis of molecular metallic barium superhydride: pseudocubic BaH12. Nat Commun 12, 273 (2021). https://doi.org/10.1038/s41467-020-20103-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-20103-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.